The European Commission has adopted a recommendation on the use of Rapid Antigen tests for the diagnosis of COVID-19. This follows the Commission’s recommendation on 28 October to ensure a common approach and more efficient testing strategies across the EU. It builds on the guidance developed with Member States input and expert advice from the European Centre for Disease Prevention and Control.
The recommendation provides guidance on how to select Rapid Antigen tests, when they are appropriate and who should perform them. It also calls for validation and mutual recognition of tests and their results. This comes ahead of the European Leaders’ virtual meeting on 19 November on the EU response to the COVID-19 pandemic. On 29 October, European Council agreed to coordinate more on testing methods.
Cross-border preparedness

The pandemic has demonstrated that there is a need to improve preparedness. Also EU needs to manage cross-border threats more effectively at both EU and Member State level. Fast and accurate testing is key for tackling COVID-19. The Commission has supported research and development of such tests. EU will have a joint procurement procedure for rapid tests. The Emergency Support Instrument provide €100 million support to Member States.
Testing tells us what the extent of the spread is, where it is, and how it develops. It is a decisive tool to slow down the spread of COVID-19.
Stella Kyriakides, Health Commissioner
Stella Kyriakides, Commissioner for Health and Food safety said: “To increase EU coordination on testing methods, we are today providing guidance to Member States on the use of Rapid Antigen test to better manage COVID-19 outbreaks. Being efficient on testing also requires having the necessary resources, which is why we are also today stepping up our support to increase Member States testing capacity. Support and solidarity is key to overcome this pandemic.”
Coronavirus test – PCR technique
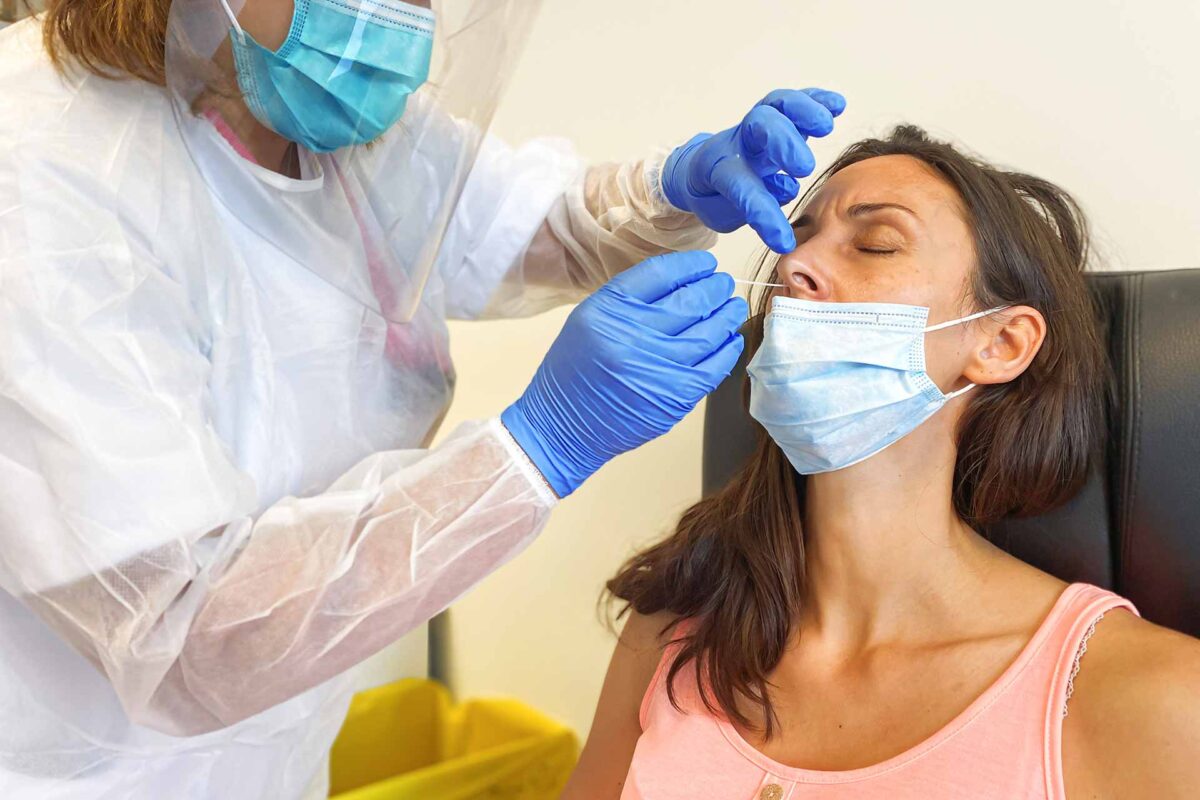
The technique used is the PCR technique. It involves taking a sample of the virus from the place where it multiplies. To detect the coronavirus, it is in the respiratory tract. The sample is therefore taken deep in the nose with a swab. The PCR test makes it possible to know if a person is contaminated at the very moment the test is carried out.
In light of the spread of the coronavirus (COVID-19), many European countries have taken almost total containment measures to combat this pandemic. Many people will have to do the test for coronavirus in the coming months.
Rapid Antigen tests
The recommendation provides guidance to Member States on the use of Rapid Antigen tests to detect the virus in specific settings. These include situations where a fast identification of infected individuals supports the management of outbreaks and regular monitoring of high risk groups. Also medical personal or in nursing homes for elderly. Member States are encouraged to conduct Rapid Antigen tests in addition to RT-PCR tests. EU States try to contain the spread of the virus, detect infections and limit isolation and quarantine measures.
EU common recognition
Mutual recognition of test results is of utmost importance in order to facilitate cross border movement, cross border contact tracing and treatment. Member States have to mutually recognise the test results for Rapid Antigen tests meeting the criteria in the recommendation carried out by authorised operating testing facilities in any EU Member States. Compliance with the recommendation may then contribute to the free movement of people and the smooth functioning of the internal market in times of limited testing capacities.

WHO supports Rapid Antigen detection tests for COVID-19 response
Rapid antigen tests are commonly used in the diagnosis of respiratory illnesses. In this case, the rapid antigen detection test looks for proteins by the SARS-CoV-2 virus. This is the virus that causes the disease called COVID-19. Antigen tests are immunoassays that detect the presence of a specific viral antigen, which means they identify people who currently have a viral infection. In some situations, they may be a viable alternative to Nucleic Acid Amplification Tests (NAAT or PCR tests), which look for viral RNA in the sample.
Rapid Antigen tests are typically used outside of laboratories, at the point of care. The turnaround time for these tests is short, 10-30 minutes, and therefore they are useful for the detection of infected contacts in an outbreak setting where urgent decisions need to be made. They can control high-risk individuals who require urgent management in locations like emergency rooms. They can also monitor trends in community epidemiology, especially among healthcare workers or other exposed cohorts.
EU countries use Rapid Antigen tests
Scientific and technical developments continue to evolve. They all offer new insights on the characteristics of the virus. Scientific researchs provide new possibilities for using different methodologies and approaches for COVID-19 diagnosis. The Commission therefore remains ready to further update the recommendation on the use of tests accordingly.
During these times of crisis, EU countries, regions and cities are stretching out a helping hand to neighbours. This assistance focus to those most in need. We meet donations of protective equipment such as masks, cross-border treatments of ill patients and bringing stranded citizens home.
This is European solidarity at its best.
EU Commission steps up actions on testing with a recommendation on Rapid Antigen tests and support to increase testing capacity.